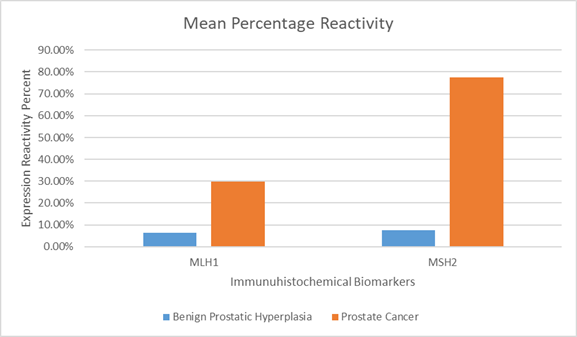
Figure 1 Mean percentage reactivity of immunohistochemistry biomarkers.
Immunohistochemical Expression of MLH1 and MSH2 in Prostate Cancer and Its Implications
1Department of Medical Laboratory Science, College of Medicine and Health Sciences, Afe Babalola University, Ado-Ekiti, Ekiti State, Nigeria*Corresponding Author: Emmanuel Omon; Email: omonea@abuad.edu.ng,
Background: Prostate cancer is a complex disease characterized by the uncontrolled growth of cells in the prostate. DNA mismatch repair (MMR) proteins, such as MutS homolog 2 (MSH2) and MutL protein homolog 1 (MLH1), play a crucial role in correcting errors in DNA replication that can lead to cancer. Loss of these proteins can result in genetic changes that contribute to the development of cancer. This study aimed to assess the expression of MLH1 and MSH2 in prostate cancer.
Methods: The study was retrospective and involved the analysis of 40 formalin-fixed paraffin-embedded tissue blocks, including 20 blocks from malignant invasive prostate cancer and 20 from benign prostatic hyperplasia (BPH) tissues. Immunohistochemical analysis was conducted using the Avidin-biotin immuno-peroxidase method to detect MLH1 and MSH2 expression. The expression levels were evaluated using a semi-quantitative method, which involved grading the intensity of staining and the percentage of stained cells per field. The stained sections were examined under a LEICA research microscope (LEICA DM750, Switzerland) equipped with a digital camera (LEICA ICC50).
Results: Nuclear staining of MLH1 was observed, with a mean positivity rate (MPR) of 6.4% in BPH cases and 29.7% in prostate cancer cases, where only 50% of cancer cells showed moderate significant expression. Nuclear staining of MSH2 was also detected, with a MPR of 7.6% in BPH cases and 77.4% in prostate cancer cases, where 60% of cancer cells showed significant marked expression. Statistically, MLH1 and MSH2 expression were significantly higher in PC compared to BPH (P<0.05), and MSH2 loss was higher compared to MLH1.
Conclusion: In conclusion, this study revealed low frequency of MLH1 immunohistochemical expression in BPH and prostate cancer. The loss of mismatch repair proteins in prostate cancer suggests a role in DNA repair processes and potential resistance to chemotherapeutic medications. As a result, defect in mismatch repair may accelerate prostate cancer development. Determining the immunohistochemical expression of the DNA mismatch repair (MMR) proteins can predict tumor behavior, serve as diagnostic markers and guide treatment decisions.
Keywords: Prostate cancer, immunohistochemistry, mismatch repair proteins, immunohistochemical expression, MutS homolog 2, MutL protein homolog 1
የጥናቱ ዳራ-የፕሮስቴት ካንሰር በፕሮስቴት ውስጥ በሚገኙ የሕዋሳት ቁጥር ከመጠን በላይ መብዛት ወይም መራባት ምክኒያት የሚከሰት ውስብስብ የበሽታ አይነት ነው፡፡ ህዋሳት ከመጠን በላይ በስህተት እንዳይራቡ የሚቆጣጠሩ ፕሮቲኖች (MLH1 እና MSH2) ሲሆኑ የእነዚህ ፕሮቲኖች በሰውነታችን ሴሎች ውስጥ በብዛት አለመገኘት ለካንሰር እድገት አስተዋጽኦ የሚያደርጉ የጄኔቲክ ለውጦችን ሊያስከትል ይችላል፡፡ የዚህ ጥናት አላማ በህዋሳት ውስጥ የሚገኙ የዘረመል እድገት ተቆጣጣሪ ፕሮቲኖችን ማለትም (MLH1 እና MSH2) በቅድመ-ካንሰር እባጭ እና በፕሮስቴት ካንሰር ሴሎች ውስጥ በምን ያህል መጠን እንደሚከሰቱ ማጥናት ነው፡፡
የጥናቱ ዘዴ፡- ጥናቱ ለህክምና አላማ የተሰበሰቡ 40 ናሙናዎችን ማለትም 20 ናሙናዎች ከቅድመ-ካንሰር እባጭ እና 20 ናሙናዎች ከፕሮስቴት ካንሰር በመውሰድ ተገቢውን የምርመራ ኬሚካል በመጨመር የMLH1 እና MSH2 ፕሮቲኖች መጠን በየትኛው የጥናት ናሙና ላይ በብዛት እንደሚገኙ የፕሮቲኖችን ኢሚኖ-ኬሚካል ባህሪ በማይክሮስኮፕ በማየት እና በድጅታል ካሜራ በማጉላት ትናቱ ተጠንቷል፡፡
የጥናቱ ውጤት፡- ከመጠን በላይ የዘረመል መባዛትን ከሚያስተካክሉ ፕሮቲኖች አንዱ (MLH1) 6́.4 በመቶ በሚሆነው በቅድመ ካንሰር እብጠት ላይ ሲከሰት 29.7 ከመቶ ደግሞ በፕሮስቴት ካንሰር ላይ ተገኝቷል፡፡ ይህም 50 ከመቶ በሚሆኑ የካንሰር ሴሎች ውስጥ ብቻ በከፍተኛ ሁኔታ ተከስቷል፡፡ በተመሳሳይ MSH2 የተባለው የፕሮቲን አይነት 7.6 ከመቶ በቅድመ ካንሰር እባጭ ላይ ሲከሰት 77.4 ከመቶ ደግሞ በፕሮስቴት ካንሰር ላይ ተገኝቷል፡፡ ፕሮቲኑ 60 ከመቶ የሚሆኑ የካንሰር ሴሎች ላይ በከፍተኛ ሁኔታ ታየቷል፡፡ ሁለቱም የፕሮቲን አይነቶች ከቅድመ-ካንሰር እባጭ ይልቅ በፕሮስቴት ካንሰር ሴሎች ላይ በብዛት እንደሚገኙ በተሰራው ምርምር ዘዴ ማረጋገጡን ተችሏል፡፡
የጥናቱ ማጠቃለያ፡- በቅድመ-ካንሰር እባጭም ሆነ በፕሮስቴት ካንሰር ሴሎች (MLH1) ፕሮቲን የመከሰቱ ሁኔታ ዝቅተኛ ነው፡፡ የዘረመል መራባትን የሚያስተካክሉ የፕሮቲን አይነቶች በብዛት አለመገኘት የበሸታውን እድገት አና ለህክምናው የሚኖረውን ምላሸ ሊጠቁም ይችላል፡፡ ጥናቱ እንደሚያሳየው የእነዚህ ፕሮቲኖች በሴሎች ውስጥ እጥረት ሲከሰት የፕሮስቴት ካንሰር እድገትን ያፋጥናል፡፡ በመሆኑም አላስፈላጊ የሴሎችን እድገት የሚገቱ የፕሮቲን አይነቶችን ማለትም MLH1 እና MSH2 ኢሚኖ-ኬሚካል ባህሪ በማጥናት የበሸታውን ምርመራና ህክምና ለማድረግ እንደአመላካች መውሰድ ይቻላል፡፡
ቁልፍ ቃላት፡- የፕሮስቴት ካንሰር፣
Prostate cancer is the most common occurring type of non-skin melanoma in males, and the second leading cause of cancer-related mortality in men, estimated to affect 1.6 million men annually and causes about 366,000 deaths1. Men with prostate cancer continue to encounter significant clinical challenges despite recent advancements in diagnosis and treatment. It is crucial to reduce unnecessary overtreatment of less severe conditions while enhancing the effectiveness of existing treatments for metastatic disease. Clinically, prostate cancer is a complex disease; some patients present with aggressive forms that progress and metastasize, while others have benign forms with minimal chances of progression. The human prostate consist of three types of cells: basal cells, which are localized to a limited extent and express biomarkers such as- cytokeratin 5 and low concentrations of androgen receptor; rare neuroendocrine cells, which are identified by the expression of endocrine biomarkers; and luminal cells, which are columnar epithelial cells that produce secretory proteins, prostate-specific antigen, differentiation antigens such as cytokeratin 8, and higher concentrations of the androgen receptor2. According to data from the International Agency for Research on Cancer (IARC), prostate cancer accounted for 29.1% of all male cancers in Nigeria in 2018. The age-standardized 1-year incidence rate was 16.1, which is less than one-fourth of the prevalence rate recorded in the United States. However, with 32.8 occurrences and 16.3 mortality per 100,000 men, prostate cancer is the most common and fatal cancer among Nigerian men3.
Like all malignancies, prostate cancer is a hereditary disease caused by tumor suppressor depression and oncogene activation. Multiple genes interacting with environmental factors lead to the complex molecular etiology of prostate cancer growth. These genetic and epigenetic changes can arise at various phases of the disease4. Numerous genetic changes are present in prostate cancer, such as point mutations, structural abnormalities, and somatic copy number or chromosomal number variations. Roughly 90% of cases of prostate cancer may have somatic copy number alterations5. It is estimated that hereditary factors account for 5–15% of prostate cancer incidences. The genes most consistently associated with prostate cancer risk are currently homologous recombination genes such as BRCA1, BRCA2, PALB2, ATM, or CHEK2 and DNA mismatch repair (MMR) genes such as MSH2, MSH6, MLH1 and PMS26.
Men with MMR gene mutations have a significantly higher risk of developing prostate cancer. The increased mutation rates and genomic instability, which can result from the dysregulation of genes associated with DNA damage repair (DDR) pathways, are often the key factors in the development of prostate cancer7. MutS homolog 2 (MSH2) and MutL protein homolog 1 (MLH1) are the mismatch repair genes most commonly mutated in this context. Unfortunately, there have been few immunohistochemical studies of DDR proteins in clinical samples of prostate cancer, and the results from various researchers have been inconclusive8-9. Identifying specific biomarkers that could indicate an increased risk of severe disease could significantly enhance the effectiveness of genetic testing. This would allow for a more personalized and successful treatment plan for patients10. This study aims to investigate the immunohistochemical expression of MLH1 and MSH2 in prostate cancer, as changes in these genes have been associated with various malignancies, including genomic instability and a higher risk of developing cancer. Investigating the immunohistochemical expression of the DNA mismatch repair (MMR) proteins can predict tumor behavior, serve as diagnostic markers and guide treatment decisions.
For this retrospective study, a total of forty (40) formalin-fixed paraffin-embedded (FFPE) tissue blocks from June 2023 to May 2024 were retrieved from the pathology archive of University College Hospital (UCH) Ibadan, Oyo State, Nigeria. The tissue samples comprised 20 blocks from malignant invasive prostate cancer and 20 blocks from benign prostatic hyperplasia (BPH). The benign group were samples with normal architecture, without evidence of cancer or high-grade prostatic intraepithelial neoplasia consistent with clinical diagnosis of BPH, while the malignant group were samples with evidence of prostate cancer, including adenocarcinoma, squamous cell carcinoma, or other malignant histologies. Hematoxylin and Eosin-stained slides were used to examine each sample. All analyses were conducted in the Molecular Laboratory, at the University of Ibadan, Oyo State, Nigeria. Clinicopathology data were gathered from the patients’ medical records. As a control, forty fresh prostate tissues and matching normal tissues adjacent to the tumor were equally collected, and frozen in liquid nitrogen, and stored at -80°C.
Formalin-fixed paraffin-embedded (FFPE) tissue blocks, obtained from patients’ biopsies; were sectioned into 4-μm-thick sections, and mounted on slides coated with aminopropyltriethoxysilane (DakoCytomation, Glostrup, Denmark) or positively charged. These sections were then rehydrated in distilled water with varying alcohol concentrations after dewaxing with xylene. The slides underwent a 3-minute antigen retrieval treatment at 121°C (15 lb) in tris-ethylenediaminetetraacetic acid buffer. Subsequently, they were incubated overnight at 4°C with 200 µl of either the MSH2 or MLH1 primary antibody (Dako, Denmark) at a 1:200 dilution (initial concentration: hMSH2 = 23.7 mg/l and hMLH1 = 78.1 mg/l). Endogenous peroxidase activity was blocked by incubating in a peroxidase-blocking solution for five minutes. After cleaning the slides with Tris-buffered saline (TBS), they were treated with two drops of polymer tagged with horseradish peroxidase and conjugated to a secondary antibody. The slides were then cleaned with TBS and incubated in 200 µl of 3,3'-diaminobenzidine chromogen for three minutes. The subsequent steps included counterstaining with hematoxylin, mounting and dehydration. BPH and lymphocytes served as internal positive controls, while normal prostate tissue acted as an external positive control. Protein expression was considered lost if nuclear staining was observed in normal prostate tissue but not in nearby cancerous cells11.
Semi-quantitative analysis was employed to determine the expression of MSH2 and MLH1. Staining intensity and percentage of stained cells per field were used to evaluate the immunoreactivity of these markers. Three levels of stain intensity were assigned: mild, moderate, and severe. The grades for the percentages of positive cells were as follows:
0.1 – 10% stained = negative (-), grade 0.
10.1 – 39% stained= positive (+), grade 1.
40.0 – 79% stained= positive (++), grade 2.
80.0 – 100% stained = positive (+++), grade 3.11
A digital camera (LEICA ICC50) connected to a LEICA research microscope (LEICA DM750, Switzerland) was used to analyze the stained sections. Digital photomicrographs were taken at different magnifications of stained sections for histomorphology and immunohistochemistry on the examined organs, and morphological changes were documented.
Figures, tables, and images (micrographs) were utilized to present the results. An electron light microscope operating at x100 and x400 was used to assess the staining of MLH1 and MSH2. Values were presented as simple frequency and percentage. Fisher Exact Test was used to determine the significant difference between variables. Statistical analysis was performed using Stata (Version 14.0, StataCorp, College Station, Texas).
The expression of MLH1 in Benign Prostate Hyperplasia (BPH) and prostate cancer was detailed in Table 1. The results indicated that in BPH, 17 slides had no significant reaction, while 3 displayed a slightly considerable reaction. In prostate cancer, 5 slides had no significant reaction, while 15 slides exhibited mild to moderate reactions. None of the slides showed marked expression of MLH1. The positivity rates were 6.4% for BPH and 29.7% for prostate cancer. The expression of MSH2 in BPH and prostate cancer was presented in Table 2. The results showed that among BPH cases, 16 slides had no significant reaction, while 4 showed mild reactions. In prostate cancer cases, 4 slides displayed mild reactions, 4 slides exhibited moderate reactions, and 12 slides showed marked reactions. The positivity rates were 7.6% for BPH and 77.40% for prostate cancer. Statistically, MLH1 and MSH2 expression were significantly higher in PC compared to BPH (P<0.05).
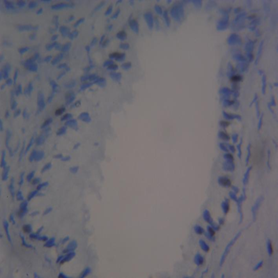
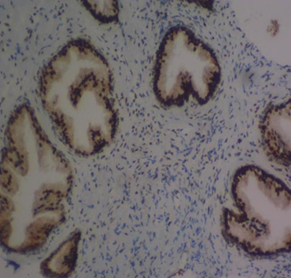
The mean percentage reactivity of immunohistochemistry biomarkers was presented in Figure 1. The results showed a gradual increase in percentage reactivity in MLH1 and MSH2 from BPH to prostate cancer. Figure 2 displayed Hematoxylin and Eosin-stained sections of control tissue samples at x100 and x400 magnification, respectively. Figure 3 presented Hematoxylin and Eosin-stained sections of MLH1 expression in BPH and PC at x100 and x400. The result of (A) showed mild expression of MLH1 in benign prostatic hyperplasia at x100, (B) exhibited moderate expression of MLH1 in prostate cancer at x100, (C) showed mild expression of MLH1 in benign prostatic hyperplasia at x400, and (D) demonstrated moderate expression of MLH1 in prostate carcinoma at x400. Figure 4 displayed Hematoxylin and Eosin-stained sections of MSH2 expression in BPH and PC at x100 and x400. The results of (A) showed mild expression of MSH2 in benign prostatic hyperplasia at x100, (B) displayed moderate expression of MSH2 in prostate carcinoma at X100, (C) illustrated mild expression of MSH2 in benign prostatic hyperplasia at x400, and (D) demonstrated moderate expression of MSH2 in prostate carcinoma at x400.
Table 1 Expression of MLH1 in indicated cases
| Groups | Total cases (n) | Neg (-) | Mild (+) | Moderate (++) | Marked (+++) | Mean Positivity Rate (%) |
|---|---|---|---|---|---|---|
| BPH | 20 | 17 | 3 | - | - | 6.40% |
| PC | 20 | 5 | 5 | 10 | - | 29.70% |
Note: MPR = (Total number of positive cells / Total number of cells evaluated) x 100*MPR of BPH vs PC: p = 0.046; Odds Ratio (OR): 0.123 (95% Cl: 0.012 – 0.995)Keys: BPH – Benign prostate hyperplasia, PC – Prostate cancer, MPR – Mean positivity rate
Table 2 Expression of MSH2 in indicated cases
| GROUPS | Total cases (n) | Neg (-) | Mild (+) | Moderate (++) | Marked (+++) | MEAN POSITIVITY RATE (%) |
|---|---|---|---|---|---|---|
| BPH | 20 | 16 | 4 | - | - | 7.60% |
| PC | 20 | - | 4 | 4 | 12 | 77.40% |
Note: MPR = (Total number of positive cells / Total number of cell evaluated) x 100*MPR of BPH vs PC: p = 0.001; Odds Ratio (OR): 0.037 (95% Cl: 0.002 – 0.065)Keys: BPH – Benign prostate hyperplasia, PC – Prostate cancer, MPR – Mean positivity rate

Figure 1 Mean percentage reactivity of immunohistochemistry biomarkers.
AB
CD
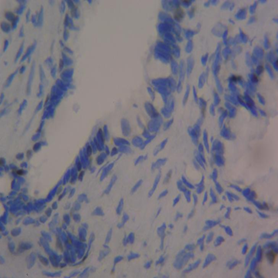
Figure 2 Haematoxylin and Eosin-stained sections (Control i.e. images refer to regions coming from normal tissue adjacent to the tumor); (A) x100 BPH (B) x100 PC (C) x400 BPH (D) x400 PC
AB
CD
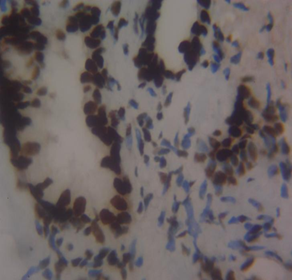
Figure 3 Haematoxylin and Eosin-stained sections of MLH1 expression in BPH and PC at x100 and x400 respectively. (A) MLH1 in benign prostatic hyperplasia at x100. (B) MLH1 in prostate cancer at x100. (C) MLH1 in BPH at x400. (D) MLH1 in prostate carcinoma at x40
AB
CD
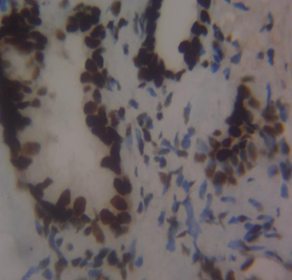
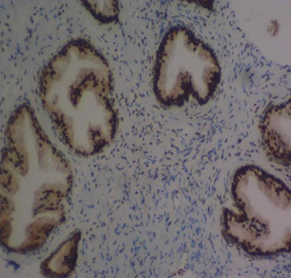
Figure 4 Haematoxylin and Eosin-stained sections of MSH2 expression in BPH and PC at x100 and x400 respectively. (A) MSH2 in benign prostatic hyperplasia at x100. (B) MSH2 in prostate carcinoma at x100. (C) MSH2 in BPH at x400. (D) MSH2 in prostate carcinoma at
The results of this study showed that in benign prostatic hyperplasia (BPH), MLH1 had negative to mild (weak) immunohistochemical expression with 85% of the sample showing negative expression, and 15% exhibiting mild expression. The mild score for MLH1 in BPH suggests a partial loss or down-regulation of MLH1 expression, which could impact the DNA mismatch repair system’s effectiveness. The negative score in our study indicates a total loss of MLH1 expression. In prostate cancer, MLH1 had negative to moderate immunohistochemical expression with 25% negative, 25% mild, and 50.0% moderate expression. This scoring system aligns with research by Wilczak et al.8 who argued against widespread intratumoral variability of MMR gene expression and suggested MMR overexpression as a common characteristic of prostate malignancies. The variation in MLH1 scores among prostate cancer patients, ranging from negative to moderate, may be due to tumor heterogeneity, stage, or genetic modifications affecting MLH1 regulation.
The mean positivity rate (MPR) of MLH1 was 6.4% in BPH cases and 29.7% in prostate cancer cases. Epigenetic changes like promoter hypermethylation or histone modifications could be causing the dysregulation of MLH1 expression in BPH. The potential damage to the DNA mismatch repair system from MLH1 loss in BPH may be less severe than in prostate cancer due to lower proliferative activity and genetic instability in benign tissues12. This aligns with previous research by Fukuhara et al.13, indicating that MLH1 is downregulated in tumors compared to benign hyperplasia and normal prostate. One possible explanation for the decrease in MLH1 expression could be a premature stop codon truncating the MLH1 gene, as reported by Chen et al.14. Fukuhara et al.13 found that MLH1 was primarily located in the nuclei of basal cells, glandular luminal epithelium, and some stromal cells in normal prostate tissue, with similar patterns in the tumor surrounding region15. In contrast, MLH1 expression was lower in prostate cancer cases compared to BPH tissue, possibly due to MLH1 gene anomalies linked to the Gleason score, Gleason pattern, and cancer aggressiveness16-17. However, neither the Gleason score nor pattern were evaluated in this study. While Javeed et al.18 found no loss of MLH1 in prostate cancer cases, Wilczak et al.8 observed MLH1 upregulation in prostate cancer subjects, which could have been influenced by sample size, methodological variations, and cancer stage. MMR proteins MSH6, PMS2, and MLH1 are often increased in cancer development due to continuous cell division and DNA replication errors19.
The study also found mild to marked immunohistochemical expression of MSH2 in prostate cancer cases and mild expression in BPH cases. Brown staining in the nuclei of prostatic epithelial cells for MSH2 indicates positive expression. The MPR of MLH1 was 7.6% in BPH and 77.4% in prostate cancer. Prostate cancer may have high MSH2 expression due to compensatory mechanisms, even in the absence of MLH1. The function of DNA mismatch repair may be partially preserved in prostate cancer cells through high MSH2 expression, despite the loss of MLH120. Guedes et al.23 documented high loss of MSH2 and gene inactivation in aggressive prostate carcinomas, while Dominguez-Valentin et al.24 concurred with these findings. Limitations of the study include a lack of genotypic analysis for MLH1 and MSH2 gene mutations and clinicopathology analyses like Gleason score and pattern.
The findings of this study revealed that the immunohistochemical expression and degree of reactivity of MSH2 in prostate cancer cases were mild in four cases, moderate in four cases, and marked in other cases. Brown staining in the nuclei of prostatic epithelial cells for MSH2 indicates positive expression. In BPH, MSH2 had mild (weak) immunohistochemical expression and degree of reactivity. The MPR of MLH1 was less than one-tenth (7.6%) in BPH cases and greater than three-quarters (77.4%) in prostate cancer. Prostate cancer may have high MSH2 expression because of compensatory mechanisms that may increase MSH2 expression even in the absence of MLH1. MSH2 may continue to function while MLH1 function is lost due to compensatory actions of other proteins in the MMR pathway20. It is well known that prostate cancer exhibits molecular heterogeneity. In some tumor regions where MLH1 is absent, MSH2 expression may be lost, while in other regions, MSH2 expression may be maintained through different mechanisms21. The function of DNA mismatch repair in prostate cancer cells may be partially preserved due to high MSH2 expression, even in the absence of MLH122. However, this may not be as effective as a fully functional MMR system found in tissues with intact MLH1 expression, potentially leading to increased genomic instability. The increase in MSH2 expression aligns with previous research8.
MSH2 loss appears more frequently in prostate cancers and has been associated with aggressive tumor characteristics. Research indicates that prostate tumors with MSH2 loss often present with high Gleason grades and increased CD8+ lymphocytes density, suggesting a robust immune response.23 This immune infiltration may contribute to the undifferentiated, high-grade appearance of these tumors. This is in agreement with previous study by Guedes et al.23 who reported the highest loss of MSH2 and gene inactivation in prostate cancer subjects. This difference may be attributed to the prevalence of high-grade aggressive prostate carcinomas, with Gleason scores ≥8 and Gleason pattern 5 (GP5), in their sample. While Dominguez-Valentin et al.24 focused on patients with Lynch syndrome and prostate cancer, they supported the findings of Guedes et al.23. However, this study had some limitations, including the absence of genotypic analysis to assess mutations in the MLH1 and MSH2 genes, and the lack of clinicopathological analyses such as Gleason's score and Gleason's pattern. Additionally, other MMR DNA repair heterodimer genes using molecular or genetic techniques were not examined, which could have further supported the findings on MLH1 and MSH2 expression in this study. Despite these limitations, our findings provide new insights into MMR gene expression and their impact on prostate cancer development and progression. Further research is needed to fully elucidate the clinical utility of MSH2 expression in prostate cancer management.
In conclusion, this study revealed low frequency of MLH1 immunohistochemical expression in BPH and prostate cancer. The loss of mismatch repair proteins in prostate cancer suggests a role in DNA repair processes and potential resistance to chemotherapeutic medications. As a result, defect in mismatch repair may accelerate prostate cancer development. MSH2 loss is associated with more aggressive disease and could serve as a marker for identifying high-risk patients.
Not applicable.
ABBREVIATIONS
AOR-Adjusted Odds Ratio
CI-Confidence Interval
COR-Crude Odd Ratio
CSA- Central Statistical Agency
EAs-Enumeration Areas
EDHS-Ethiopian Demographic and Health Survey
USAID -United States Agency for International Development
Ethical approval for this study was obtained from the Health Research Ethics Committee, College of Medicine and Health Sciences, Afe Babalola University, Ado-Ekiti, Ekiti State, Nigeria. Informed consent was obtained from each participant who participated in the study. All experiment in this study was performed in accordance with the Declaration of Helsinki.
Not applicable.
The data generated in the present study may be requested from the corresponding author.
The authors declare no financial or non-financial competing interests.
The authors did not receive any funding for this research.
V.O.E conceptualized the manuscript. The methodology was developed by T.F. Formal analyses and visualization were conducted by E.A.O. Resources were contributed by all authors. E.A.O and T.F. wrote the original draft, while review and editing was done by all authors. Supervision was provided by V.O.E. E.A.O. was responsible for coordination with the co-authors and submission of the article. All authors have read and approved the final manuscript.